The place did all these advanced intracellular buildings come from?
Greater than 1.5 billion years in the past, one thing momentous occurred: two tiny primitive cells grew to become one. Maybe greater than some other occasion—besides the origin of life itself—this merger radically modified the course of evolution on our planet.
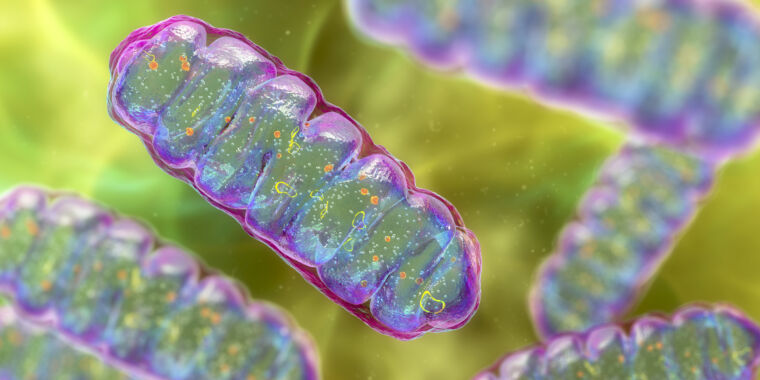
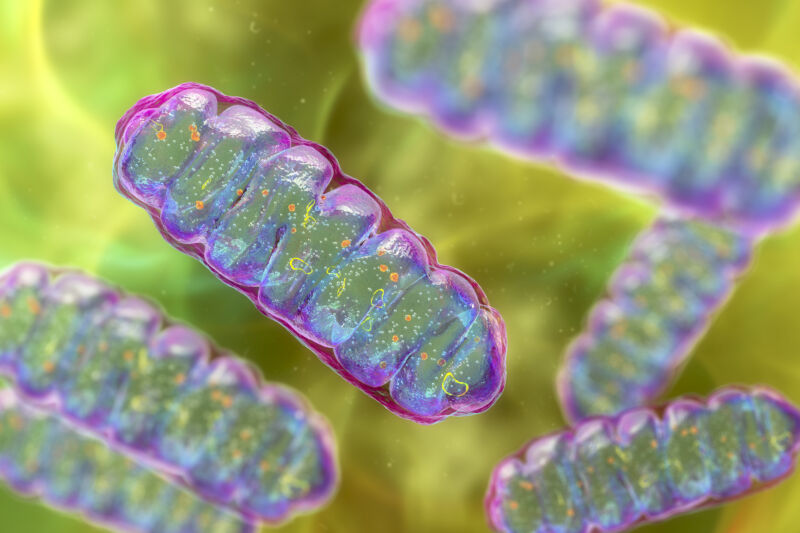
One cell ended up inside one other and developed right into a construction that schoolchildren be taught to confer with because the “powerhouse of the cell”: the mitochondria. This new construction supplied an incredible energetic benefit to its host, a prerequisite for the following evolution of advanced, multicellular life.
However that is solely a part of the story. the Mitochondria It’s not the one necessary construction inside advanced eukaryotic cells. There’s a membrane-bound nucleus, guarding the Genome. There’s a entire system of inside membranes: the endoplasmic reticulum, the Golgi equipment, lysosomes, peroxisomes, and vacuoles—essential for making, transporting, and recycling proteins and different cargo in and across the cell.
The place did all these buildings come from? With occasions misplaced within the deep previous and few traces that can be utilized as evolutionary proof, that is a particularly troublesome query to handle. Researchers have proposed numerous hypotheses, however solely not too long ago, with some new instruments and methods, have cell biologists been capable of examine the beginnings of this advanced construction and shed some gentle on its potential origins.
Microbial fusion
The concept that eukaryotes arose from the fusion of two cells dates again greater than 100 years, but it surely didn’t develop into accepted or identified till the Nineteen Sixties, when the late evolutionary biologist Lynn Margulis articulated her concept of endosymbiosis. Margulis mentioned mitochondria possible originated from a category of microbes often called alphaproteobacteria, a various group that right this moment contains the micro organism liable for typhus and different micro organism necessary within the genetic engineering of crops, amongst many different issues.
Nothing is understood concerning the nature of the unique host cell. Scientists advised that it was really fairly advanced, with quite a lot of membranous buildings inside it. Such a cell would possibly be capable to swallow issues — a posh and energy-expensive characteristic in eukaryotes known as phagocytosis. This can be how mitochondria first entered the host.
However this concept, known as the “late mitochondria” speculation, doesn’t clarify how or why the host cell grew to become advanced to start with.
In 2016, evolutionary biologist Bill MartinCell biologist Sven Gold And the world of bioinformatics Sriram GargResearchers from the College of Düsseldorf in Germany have proposed a very totally different mannequin often called the “early mitochondria” speculation. They argued that as a result of no primitive cells right this moment comprise any inside membrane buildings, it’s unlikely {that a} cell would have contained such buildings greater than 1.5 billion years in the past.
As a substitute, scientists thought that the endomembrane system—an entire patchwork of components discovered inside right this moment’s advanced cells—might have advanced quickly after the alphaproteobacterium settled inside a comparatively easy host cell, from a species in a category known as archaea. Membranous buildings could come up From blebs, or vesicles, released by the mitochondrial progenitor.
Gould, Garg, and Martin be aware that free-living micro organism secrete vesicles on a regular basis, for all types of causes, so it appears affordable to assume that they might proceed to take action when confined inside a bunch.
Ultimately, these vesicles grew to become specialised for the features that membrane buildings carry out right this moment inside eukaryotic cells. They could even fuse with the host cell membrane, which helps clarify why the eukaryotic plasma membrane comprises lipids with bacterial traits.
The biochemist says the vesicles might carry out an necessary major operate Dave Spiger From the College of Amsterdam. The brand new endosymbiont was speculated to generate a lot of poisonous chemical compounds known as reactive oxygen species, by oxidizing fatty acids and burning them for vitality. “It destroys all the things, it is poisonous, particularly contained in the cell,” Spiger says. Sequestering them inside vesicles would have helped hold the cell secure from hurt. He says.
Gould, Garg, and Martin add that there was one other drawback created by the brand new visitor that might have been solved by making membrane boundaries. After the arrival of Alphaproteobacterium, components of its DNA grew to become blended with the host archaeal genome, ensuing within the disruption of necessary genes. Fixing this meant creating machines to splice these overseas items – identified right this moment as introns – from… Messenger RNA transcription of genesso the protein making directions won’t be distorted.
However that created one other drawback. The protein-making equipment – the ribosome – works in a short time, linking collectively many amino acids per second. In distinction, the cell’s intron removing system is gradual, reducing out about one intron per minute. So, except the cell can hold the mRNA away from the ribosomes in order that the mRNA is processed correctly, the cell will produce many irrational and ineffective components Proteins.
The perinuclear membrane supplied a solution. Performing as a spatial barrier, it permits mRNA splicing to terminate within the nucleus earlier than the intron-free mRNA is translated into the cell’s inside fluid, the cytosol. “That is the selective strain behind the origin of the nucleus,” says Martin. To kind it, the vesicles secreted by the endosymbiont could also be flat and wrapped across the genome, making a barrier to maintain ribosomes out, however nonetheless permitting small molecules to go freely.